Engineering Protein-Polymer Hybrids
Engineering Protein-Polymer Hybrids
The field of protein-polymer conjugation began because the properties of the protein are improved when polymers are attached. It has been shown that attaching polymers increases protein solubility, stability, and biodistribution, while decreasing immunogenicity. The key advances in protein-polymer conjugation have focused on developing the ability to site-specifically attach the polymer to protein, and then graft the polymer from the protein. Site-specific control over the location of the polymer on the protein prevents heterogeneity, which ensures that neither the structure nor the activity of the protein is compromised. Grafting the polymer from the protein prevents the steric problems associated with coupling two large molecules of differing properties; it greatly simplifies purification of the new protein-polymer hybrid because the low molecular weight residual monomer can be easily removed. Using genetically encoded unnatural amino acids, the Mehl lab has developed the only general method to site-specifically incorporate a polymer initiator into a protein, which facilitates efficient grafting of polymers from the protein Figure 1.
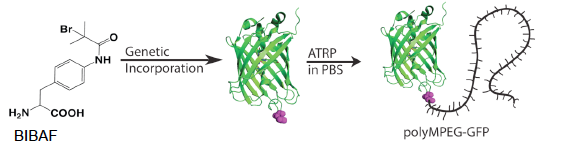
Figure 1. Translational components were evolved to genetically incorporate synthesized radical initiator BIBAF into proteins. Under standard ATRP conditions polymers were grown from initiator containing proteins without compromising protein structure. JACS 2010 (132) 13575.
An obvious protein polymer-hybrid use, which is being exploited by many companies, is the preparation of stabilized protein-based therapeutics, biosensors, bioreactors, and diagnostic devices. However, this new ability to site-specifically control the growth of many polymers from many different proteins opens up new research areas, in which the fabrication and structurally responsive characteristics of polymers can be efficiently combined with the enzymatic and structural aspects of biology. We are exploiting our ability to site-specifically grow polymers on proteins to develop two areas: (1) fabrication of catalytic nanoparticles for therapeutics and diagnostics, simultaneously allowing determination of restricted molecular motion on stabilizing proteins and (2) construction of smart protein-polymer hybrids that regulate catalytic activity or binding interactions based on a response to physical or chemical stimuli. With this technology developed, we are organizing proteins in compartments or locations and in 3D space mimicking the organization of living cells, thereby elucidating how they direct the flow of chemical information. A significant advance would be to mimic the complex chemical processes of a cell with well-defined materials by organizing different proteins in the 3D space of the fabricated polymer material. By layering materials that contain a series of regulatable protein-polymers, complex systems can be designed that will direct chemical processes similar to electron transport, metabolism, and photosynthesis.
1. Nanogels
Currently, we are growing polymers from catalytic proteins that allow construction of stable, catalytic nanoparticles. Restricting the molecular motion of proteins is a classic method for improving protein stability; however, it has been challenging to rigorously assess of the method due to an inability to construct well-defined homogeneous materials with proteins. Because we can efficiently vary the site of attachment, the extent to which the location of attachment on the protein surface is affecting stability can be separated from how much the nanoparticle matrix is stabilizing the protein toward harsh conditions. To develop the field of protein-nanogels understanding the stabilization imparted by different types of nanogels matrixes will be imperative.
To explore protein-nanogels, we generated nanogels from two proteins, GFP and catalase, using inverse mini-emulsions in water containing polysorbate emulsifier (span 80) and cyclohexane. GFP will be effective for assessing nanogel size and testing levels of protein incorporation and structural stability, since GFP fluorescence is linked to its structural integrity (Figure 2).
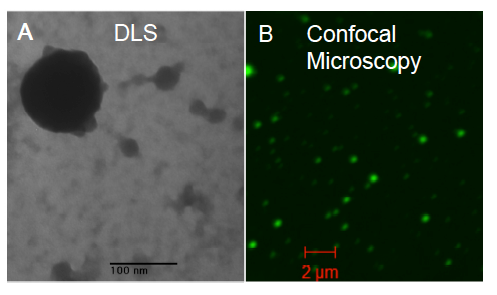
Figure 2. Nanogels formed using GFP-BIBAF as an initiator. (A) Dynamic light scattering can be used to confirm the size of protein-nanogels. (B) Confocal Microscopy can verify that nanogels contain attached GFP and determine when it unfolds due to loss of fluorescent signal.
The nanogels we generated using catalase-BIBAF have been shown to have comparable catalytic activity as free catalase-BIBAF for generating oxygen from hydrogen peroxide. Catalase is a functional homotetramer, meaning that each structure has the potential of connecting to the gel from four initiation sites. These studies on protein nanogels will allow us to design protein-nanogels for protein delivery, as the gels can be designed to break down in vivo. Our studies on protein-nanogel stability will also apply to spin coating surfaces with protein-hydrogels, since the synthetic procedures are similar. This will allow for coating implantable devices and diagnostic devices with stabilized protein-hydrogels.
In order to more easily control polymer growth from proteins and release of proteins from nanogels, we genetically encoded hydrolysable initiators. These initiators afford greater control over polymer growth and also allow slow release of protein from protein nanogels as an additional drug delivery approach.
2. Smart polymers
A large number of proof of concept papers have shown that polymers can exhibit large property changes in response to small physical or chemical stimuli. Table 1 shows the wide range of stimuli that have been used to alter stimuli responsive polymers, or “smart polymers.”
Stimuli for Smart Polymers
Temperature changes pH changes
Redox changes Electrolyte concentration changes
Metabolic intermediates (e.g. glucose) Enzyme activity (peptidases)
Light Antigen/antibody interactions
Electrical or magnetic field changes Sonic energy changes
Table 1. Stimuli that can induce a large property changes to polymers.
Additionally, these polymers can be attached to proteins used to regulate catalytic activity or binding interactions (Figure 3). A key to controlling these smart polymer-protein hybrids is the ability to vary the site of the polymer on the protein and the length of the polymer, allowing for tuning of equilibrium.
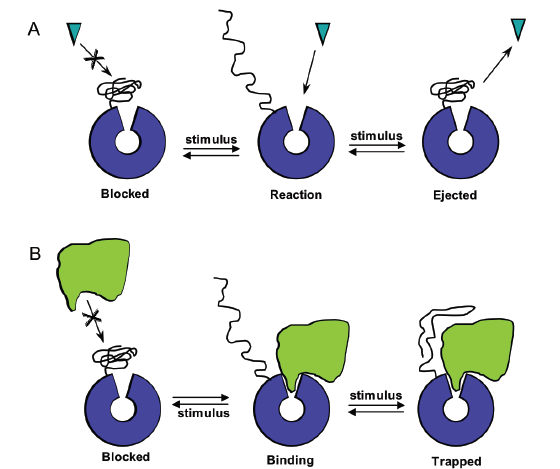
Figure 3. Illustration of the use of properly positioned smart polymers to regulate proteins or protein interactions upon applying a stimulus (such as a change in temperature, pH, or light). (A) Smart polymers can block an active site or release a bound product. (B) Smart polymers can prevent protein binding interactions or trap a protein interaction.